influenza human H3N2 NP¶
Construction of a mutational path through protein sequence space for the nucleoprotein (NP) of human H3N2 influenza.
This analysis is found in the ./examples/influenza_human_H3N2_NP/ subdirectory in the mutpath package on GitHub.
This analysis essentially recapitulates that in Gong et al, 2013, although it is not the exact code used for that paper and so may not be a completely precise match.
This analysis was performed by Jesse Bloom.
Input sequence data¶
The beginning data files in this directory are as follows:
NPhumanH3N2.fasta is a FASTA file of all full-length coding nucleotide sequences from human H3N2 influenza, excluding lab strains, as downloaded from the Influenza Virus Resource on May-19-2011.
Aichi1968_NP.fasta and Brisbane2007_NP.fasta are FASTA files containing the coding sequences from the NP genes from the A/Aichi/2/1968 and A/Brisbane/10/2007 H3N2 strains, as encoded in the Bloom lab reverse genetics plasmids pHWAichi68-NP (#593) and pHWBR10-NP (#589).
Generation of BEAST input XML file¶
The BEAST XML files were generated as follows using the mutpath_parse_to_beastxml.py script on my personal computer.
Input file mutpath_parse_to_beastxml-prot-infile.txt was created manually.
A files to specify excluded outlier sequences was created and named excluded_prots.txt.
The script was then run:
mutpath_parse_to_beastxml.py mutpath_parse_to_beastxml-prot-infile.txt
This created the .txt and .pdf files with prefixes prot-divergence_gaps / prot-divergence_identities. These files were used to identify likely outlier sequences, which were added to the excluded_prots.txt file, and the script was run again.
The final output files were:
prots.fasta
prots.nex
prots.xml
The XML files is the BEAST input file for the next step.
Running BEAST¶
BEAST was used to run the prots.xml file. The version of BEAST used was v1.8.0pre Prerelease r5356, using the BEAGLE (revision 1093) library. This was done on the FHCRC’s rhino computing cluster.
Six separate directories were created called prots_BEASTrun_1, prots_BEASTrun_2, ... The prots.xml file was copied into prots_BEASTrun_*. BEAST was then run in each of these directories. The runs were initiated by sbatch as described below. Note that it specifies the BEAGLE library location based on the fact that these have been installed locally in /home/jbloom/BEAGLE_libs.
Specifically, to run BEAST, the following file was created with the name runcommand.sbatch:
#!/bin/sh
#SBATCH
#PBS -l walltime=480:00:00
echo "Starting..."
java -Xmx4048m -Xms4048m -Djava.library.path=/home/jbloom/BEAGLE_libs/lib -cp ~/BEAST/build/dist/beast.jar dr.app.beast.BeastMain -beagle prots.xml > screenlog.txt
echo "Finished."
The file was run using:
sbatch runcommand.sbatch
This created the BEAST .trees and .log files.
The output directories were then copied back to my local computer for analysis. However, these directories are not included in the repository on GitHub due to the large file size.
The .log files were examined using Tracer to check for MCMC convergence.
Using a burn-in of the first 2 million steps (first 10% of steps), the effective sample size (ESS) for both the posterior and the root height exceed 200, suggesting good MCMC convergence.
Compacting the .trees files¶
The prot.trees files created by running BEAST were then compacted to new files called prots_compact.trees using the mutpath_compact_trees.py script.
The commands (run inside each individual run directory were as follows):
% du -h prots.trees
939M prots.trees
% mutpath_compact_trees.py prots.trees
% du -h prots_compact.trees
40M prots_compact.trees
% rm prots.trees
Extracting the mutational paths¶
To extract the mutational paths and merged trees from the compacted .trees file, the script mutpath_get_paths.py was used. The first 10% of each BEAST run was specified as burnin. The file mutpath_get_prot-paths_infile.txt was created manually. The script was then run using the following command:
mutpath_get_paths mutpath_get_prot-paths_infile.txt
to extract the protein mutational paths. This created an output file listing the mutational paths (prot_mutpaths.txt) as well as the merged trees without branch annotations (prot_trees_merged.trees). The merged .trees file is designed for building maximum-clade credibility trees with TreeAnnotator.
Making and annotating the maximum clade credibility tree¶
The maximum clade credibility tree was constructed from the prot_trees_merged.trees files built by mutpath_get_trees.py using TreeAnnotator (version 1.6.1). The commands was:
~/BEASTv1.6.1/bin/treeannotator prot_trees_merged.trees prot_maxcladecredibility.trees
to created the maximum clade credibility tree prot_maxcladecredibility.trees.
This files was then further annotated with mutpath_annotate_tree.py. An input file for this script was created and named mutpath_annotate_prot-tree.txt, and then the script was run with:
mutpath_annotate_tree.py mutpath_annotate_prot-tree.txt
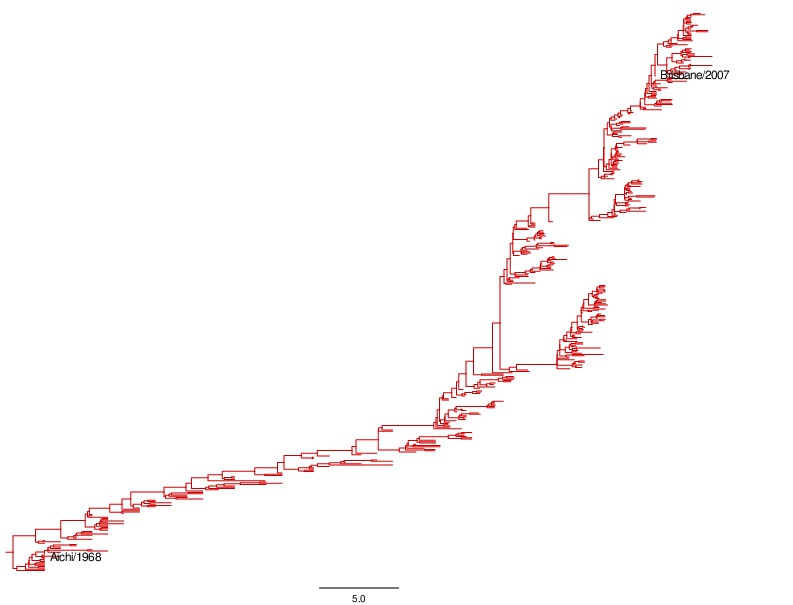
This created the file annotated_prot_maxcladecredibility.trees. FigTree was used to visualize this file, manually recolor the branches red, and save the image annotated_prot_maxcladecredibility.pdf. This PDF image was used to create annotated_prot_maxcladecredibility.jpg using the shell convert utility.
Making the mutational trajectory and dating the mutations¶
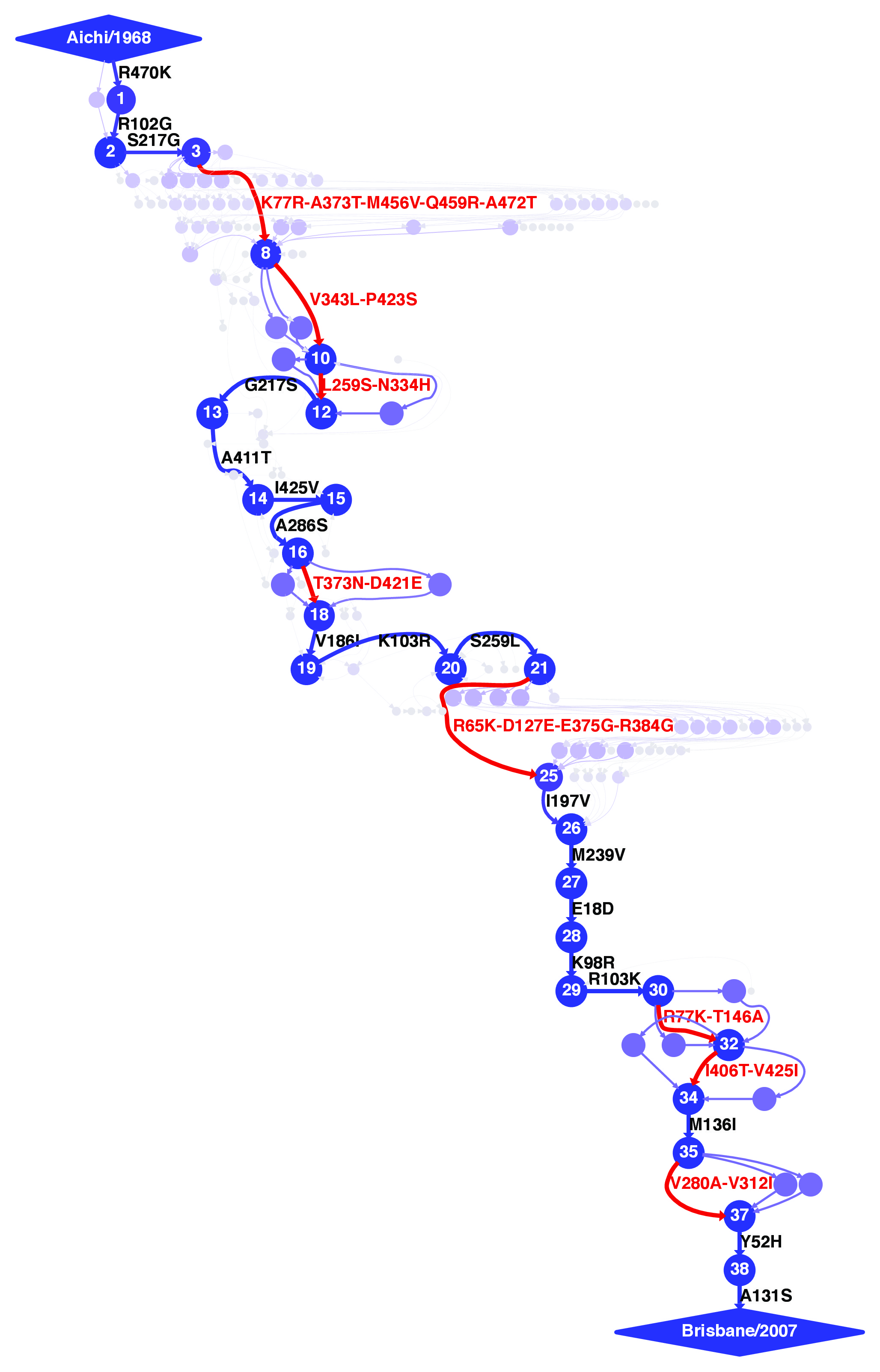
The mutational trajectory was created with mutpath_make_digraph.py from the prot_mutpaths.txt mutational paths file created by mutpath_get_paths.py. The input file mutpath_make_digraph-prot_infile.txt was created manually, as was the file nodenames.fasta, which defines the names of high-confidence nodes as used in “Stability-mediated epistasis constrains the evolution of an influenza protein.” The mutpath_make_digraph.py script was then run on these input files with:
mutpath_make_digraph.py mutpath_make_digraph-prot_infile.txt
This created a mutational trajectory in protein sequence space. The trajectory was written in the DOT language in the file prot_trajectory.dot. It was then visualized using GraphViz (version 2.30), which was also used to save the image files prot_trajectory.pdf and prot_trajectory.jpg. The script also created the following additional output files which contain information about the mutation dates and node persistence times:
prot_mutationdates.pdf
prot_mutationdates.txt
prot_nodepersistence.txt
The prot_mutationdates.pdf file was used to create prot_mutationdates.jpg using the shell convert utility.